[ AsiaOne / Straits Times ] Singapore inks deal for Merck antiviral pill to treat Covid-19 and its variants
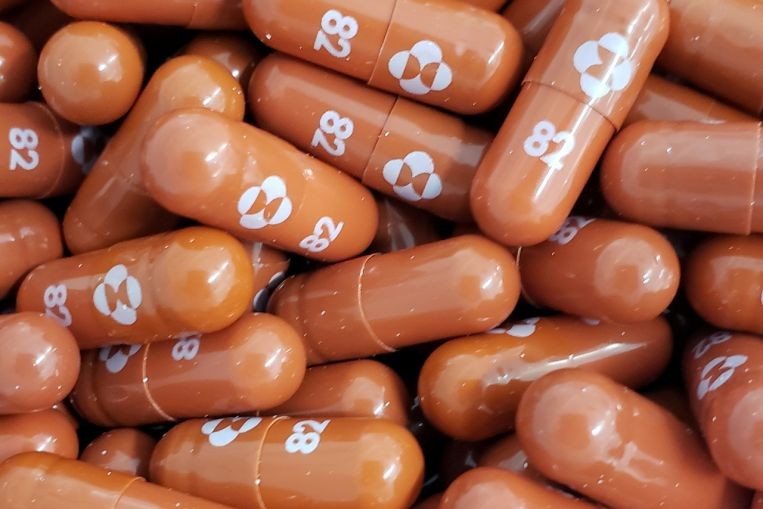
The Republic has inked a supply and purchase agreement for an antiviral pill to treat Covid-19 that is said to be effective against all known variants of the virus, including the Delta variant.
The drug was developed by pharmaceutical firm Merck in the United States and Canada, together with Miami-based Ridgeback Biotherapeutics. Merck is known as MSD elsewhere in the world.
The drug will be available in Singapore once it has received authorisation and approval for use, said MSD in an announcement on Wednesday (Oct 6).
This particular enzyme — known as the viral polymerase — is conserved across different variants, making molnupiravir effective across the Gamma, Delta and Mu variants.
Data from clinical trials suggests the drug is most effective when given early in the course of infection, said MSD.
Interim trial results last Friday showed the drug may reduce the chance of hospitalisation or death by half for patients who are at risk of severe disease.
The companies plan to seek US emergency use authorisation for the pill as soon as possible, and to submit applications to regulatory agencies worldwide.
So far, Australia has entered advanced supply agreements with MSD to purchase 300,000 courses of the drug.
Other places like South Korea, Thailand, Taiwan and Malaysia are also in talks with the company to purchase the drug.
In anticipation of the results, MSD expects to produce 10 million courses of treatment by the end of the year, with more doses to be produced next year.
The second trial, looking at molnupiravir as a preventative drug, will study the efficacy and safety of administering the drug to prevent the spread of Covid-19 within household settings.
Results are likely to be available in the first half of next year.
Local researchers had in their own study also identified molnupiravir as an effective drug against the original Sars-CoV-2 virus, as well as the Beta and Delta variants.
A team led by Professor Dean Ho, director of the National University of Singapore’s Institute for Digital Medicine, together with the DSO National Laboratories, found that molnupiravir, combined with baricitinib, an anti-inflammatory drug, could be a possible combination for Covid-19 treatment.
Prof Ho had told ST in August that both drugs come in a pill format and can therefore be administered to those with mild illness who are recovering at home, or in a community care setting.